- 7BIOM037W Systems Biology Coursework 1 Report 2026 | University of Westminster
- NCFE CACHE Level 3 Unit 8 Professional Practice Portfolio 1 Coursework 2026
- NCFE CACHE Level 3 Unit 9 Supporting Emergent literacy Coursework 2026
- 6WBS0035/ 6WBS0036 Digital Economy CW1 Assignment Brief 2026 | UOH
- DSM060 Data Science Research Topics Coursework Assignment 2026 | UOL
- BARC0087 Structures Materials & Forming Techniques Coursework 2026 | UCL
- LL5306 Commercial Law Assessment Coursework Brief 2026 | Kingston University
- M22319 / M33098 Numerical Skills & Economics Assessment Coursework | UOP
- BMG872 Global Strategy Development and Implementation Individual Assignment CWK Brief 2026
- LLB020N204A Law of Property Assessment Coursework Brief 2026
- BS3397 Microeconometrics Coursework Assignment Brief 2026 | AU
- UMAD47-15-M Managing Finance Assessment Coursework Brief | UWE
- BST851 Business Data Analytics Assessment Coursework 2026
- MMM143 International Business and the World Economy Coursework 2026
- EMS402U Engineering Design Coursework Project Report 2026 | QMUL
- 25BSC565 Fundamentals of Strategic Management Coursework Brief
- MARK5025 Contemporary Marketing Communications Assessment Coursework Brief 2
- GEEN1127 Design and Materials Individual Coursework Brief 2025-2026 | UOG
- LD7098 Cyber Security Principles Coursework Assessment 2025-26 | Northumbria University
- EG7004 Soil Structure Engineering Assignment 1 Coursework Semester A 2025/26 | University of East London (UOEL)
BPS319 PBL 3 Natural Product Chemistry vs Kinetic Isotope Effects Coursework | UOL
| University | University of Liverpool (UOL) |
| Subject | BPS319 Natural Product Chemistry vs Kinetic Isotope Effects |
BPS319 PBL 3 Coursework
In this module, we studied the Woodward synthesis of cholesterol, which consists of 35 linear steps. Since the 1950’s when this total synthesis route was published, there has been much interest in the development of improved synthetic routes towards steroid compounds.
The diketone molecule shown below, represents a key intermediate in the total synthesis of numerous biologically relevant natural products, including steroids.
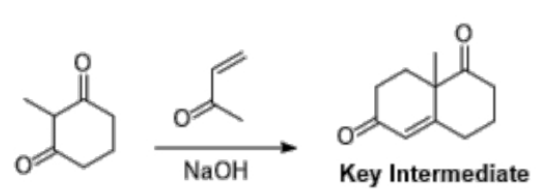
a) Draw a reaction mechanism to show the formation of the key intermediate shown, from 2-methyl-1,3-cyclohexanedione and 3-buten-2-one under basic conditions. (7 marks)
The above reaction results in the formation of a new stereogenic centre. An asymmetric synthesis which uses L-Proline as an organocatalyst affords the stereoisomer shown below in 76% ee.
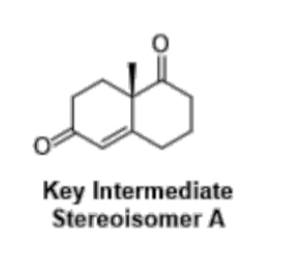
b) Using the Cahn-Ingold-Prelog rules, assign the stereochemistry of the enantiomer shown. Show your full working by drawing an annotated structure. (1 mark)
c) Given that the enantiomeric excess is 76% in favour of the enantiomer drawn, what is the relative proportion of each enantiomer? Show your full working. (2 marks)
d) With full justification identify the rate determining step (RDS) within your Robinson Annulation mechanism, highlighting it in your mechanism from part a. (4 marks)
e) Design and draw an isotopically labelled reactant that could corroborate your choice of RDS. (2 marks)
f) What type of Kinetic Isotope Effect and relative reaction rate would be seen for your labelled substrate if your choice of RDS is correct? Justify your answer. (4 marks)
Struggling for BPS319 Natural Product Chemistry vs Kinetic Isotope Effects CW?
Many UOL students find BPS319 PBL 3 tough—mechanisms, stereochemistry, isotope labelling and KIE calculations are tricky and time-consuming. If you need a clear, human-written solution that follows the assignment rubric, Students Assignment Help delivers custom, plagiarism-free answers written by chemistry experts. We cover mechanism annotation, stereochemical assignments, percent-ee calculations, RDS justification and isotope design. Check our Chemistry Assignment Help for subject-specific examples, or use assignment help uk for full service options.




